Non-Surgical Reusable Isolation Gown Level 2
RECOMMENDED USE:
DO NOT use (I) in a surgical setting; (II) where significant exposure to liquid bodily or other hazardous fluids may be expected; or (III) in a clinical setting with high risk of infection.
Description
Non-Surgical, Reusable Isolation Gowns are a cost conscious and environmentally responsible choice that provides protection to the wearer from the transfer of microorganisms and bodily fluids in low- or minimal-risk patient isolation situations and that are not intended for use during surgical procedures, invasive procedures, or when there is a medium or high risk of contamination.
As per current FDA Enforcement Policy for Gowns, Other Apparel, and Gloves During the Coronavirus Disease (COVID-19) Public Health Emergency Guidance for Industry and Food and Drug Administration Staff, published March 2020, this gown may be used as a level 3 gown where an FDA cleared level 3 gown is unavailable.
Features
- Meets the FDA and CDC guidelines for a Level 2 isolation gown.
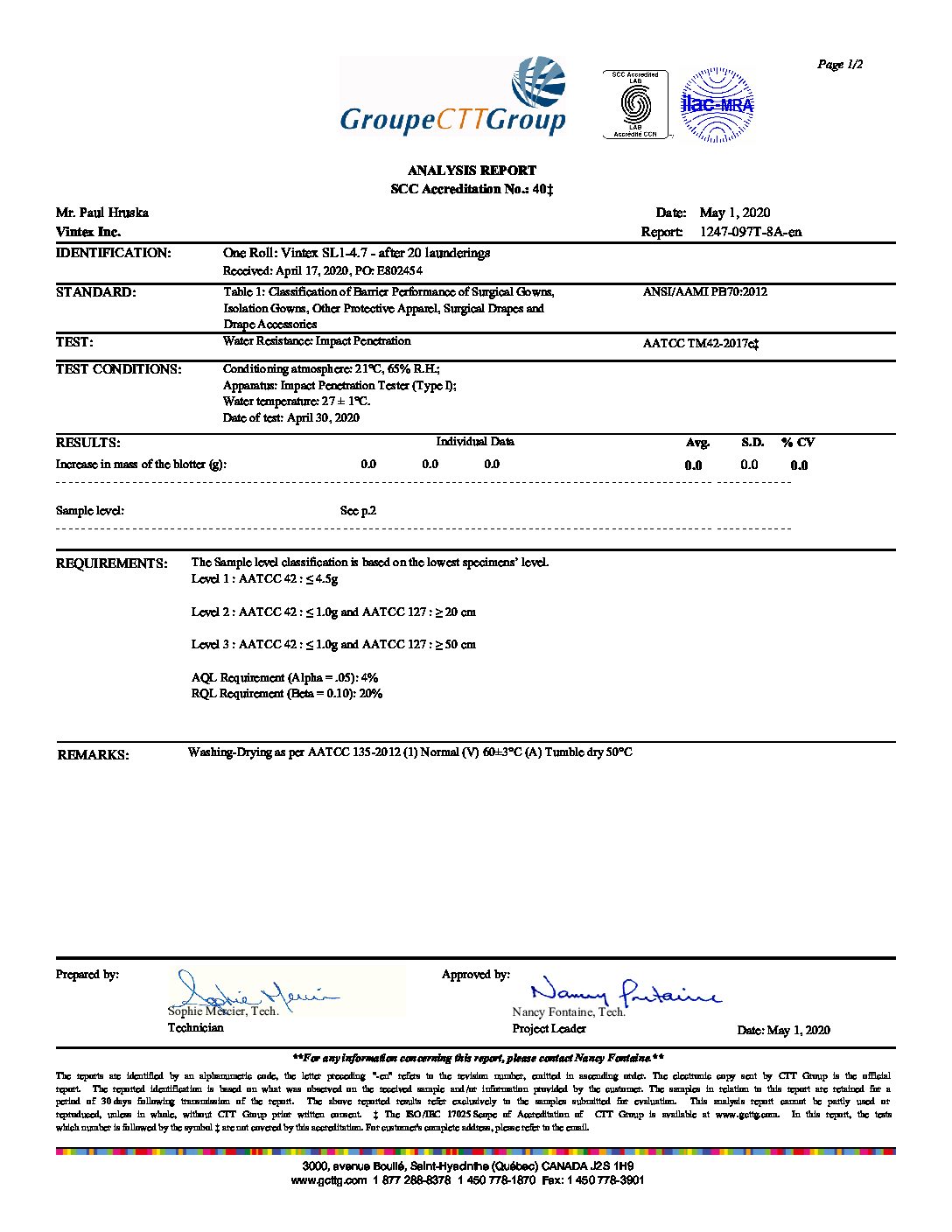
- Soft, flexible V-Care barrier fabric tested to meet AAMI / ASNSI PB 70 fluid barrier requirements for Level 3.
- Poly/vinyl fabric is environmentally friendly – PROP65-free, phthalate-free.
- Fabric maintains level 3 barrier up to 25 times commercial laundering.
- Overlap back with twill tie neck and back waist closures.
- Generously sized with longer sleeve length for comfortable arm movement with white ribbed cuffs.
- Reusable gown may be washed and dried – see washing instructions.
- Non-sterile.
Minimum order 10 units
See Image Gallery